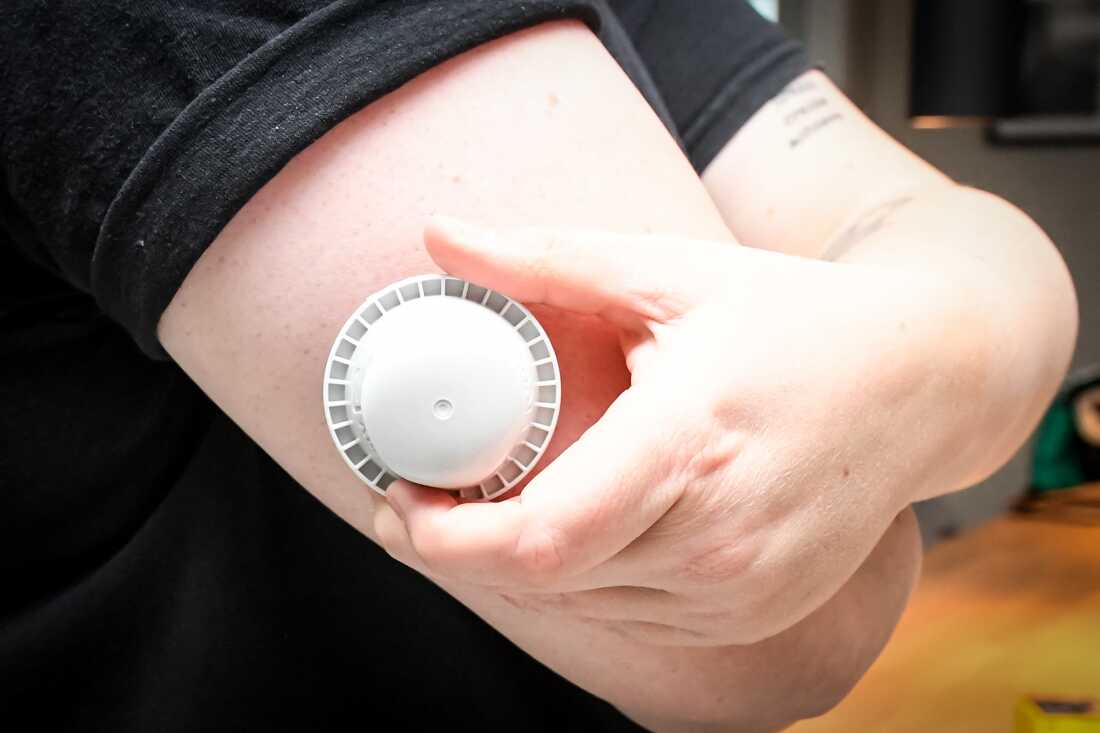
A photograph illustration reveals the applicator of the Abbott Freestyle Libre 3 Plus glucose sensor on the arm of a affected person on Thursday.
Jill Delsaux/Getty Pictures
cover caption
toggle caption
Jill Delsaux/Getty Pictures
Abbott Diabetes Care has warned of defective glucose readings on a few of its gadgets, doubtlessly linked to seven deaths and tons of extra critical accidents.
Some 3 million of the corporate’s FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors have been affected by the difficulty that was decided by inside testing to have resulted on a single manufacturing line. About 1.5 million of these gadgets are estimated to have expired or been used, Abbott stated.
In a press launch, the corporate stated “inside testing decided that some sensors might present incorrect low glucose readings.” Prospects who verify their machine is affected “ought to instantly discontinue use and get rid of it,” Abbott stated.
“If undetected, incorrect low glucose readings over an prolonged interval might result in incorrect remedy choices for folks dwelling with diabetes, corresponding to extreme carbohydrate consumption or skipping or delaying insulin doses,” the corporate stated. “These choices might pose critical well being dangers, together with potential harm or loss of life, or different much less critical problems.”
Abbott stated it continues to provide sensors via the method and the corporate didn’t anticipate vital provide disruptions on account of the difficulty.
Of the seven individuals who died, all have been situated outdoors of the US. And of 736 “extreme” accidents, 57 occurred throughout the U.S.
Diabetes is a illness that impacts your physique’s manufacturing of and response to the hormone insulin. Glucose monitoring helps folks dwelling with diabetes to find out their blood sugar and make choices on meals consumption and drugs.
Abbott stated prospects utilizing the doubtless affected sensors ought to go to FreeStyleCheck.com to find out the standing of their machine. The corporate stated it’s going to substitute defective sensors at no cost.
The Meals and Drug Administration has extra info on the recall.

